Thalidomide
Our responsibility today
The Thalidomide tragedy will always remain a part of our company’s history. We will never forget what happened, and we deeply regret the severe consequences for those affected and their families. We take our responsibility to help these people very seriously. Our Grünenthal Foundation supports affected individuals and families by funding projects that contribute to a more independent life. Through the foundation, we aim to provide help where it is most needed and remain in close contact with people whose lives have been impacted by Thalidomide.
In November 2021, Dr. Michael Wirtz, shareholder of Grünenthal, apologised to those affected and their families on behalf of his family. From many conversations, we appreciate how important this personal statement was to this community. We therefore welcome this gesture as a further step on the chosen path of dialogue between affected people, Grünenthal and the shareholder family.The effects of the tragedy can still be felt today. We are committed to keeping the memory alive by continuously expanding the information we make available.
Background information on Thalidomide can be found on our website www.thalidomide-tragedy.com.
Further information on the Grünenthal Foundation can be found here: www.grunenthal-foundation.com.
Grünenthal Foundation for the support of Thalidomide-affected people
The Grünenthal Foundation was established in the beginning of 2012 and integrated the “Hardship Initiative” founded by Grünenthal in 2011. Its mission is to improve quality of life for people affected by a Thalidomide. Personal communication with people affected by the tragedy is the key to the Foundation’s work.
The team takes time to learn about each individual’s needs and then makes sure that the Foundation offers support in ways that make a real positive impact. So far, the Grünenthal Foundation has supported more than 700 affected people and approved more than 3,450 individual applications. Additionally, the Foundation has implemented various national and international projects.
Visit the Grünenthal Foundation‘s website for more informationThe Contergan Foundation for Disabled People
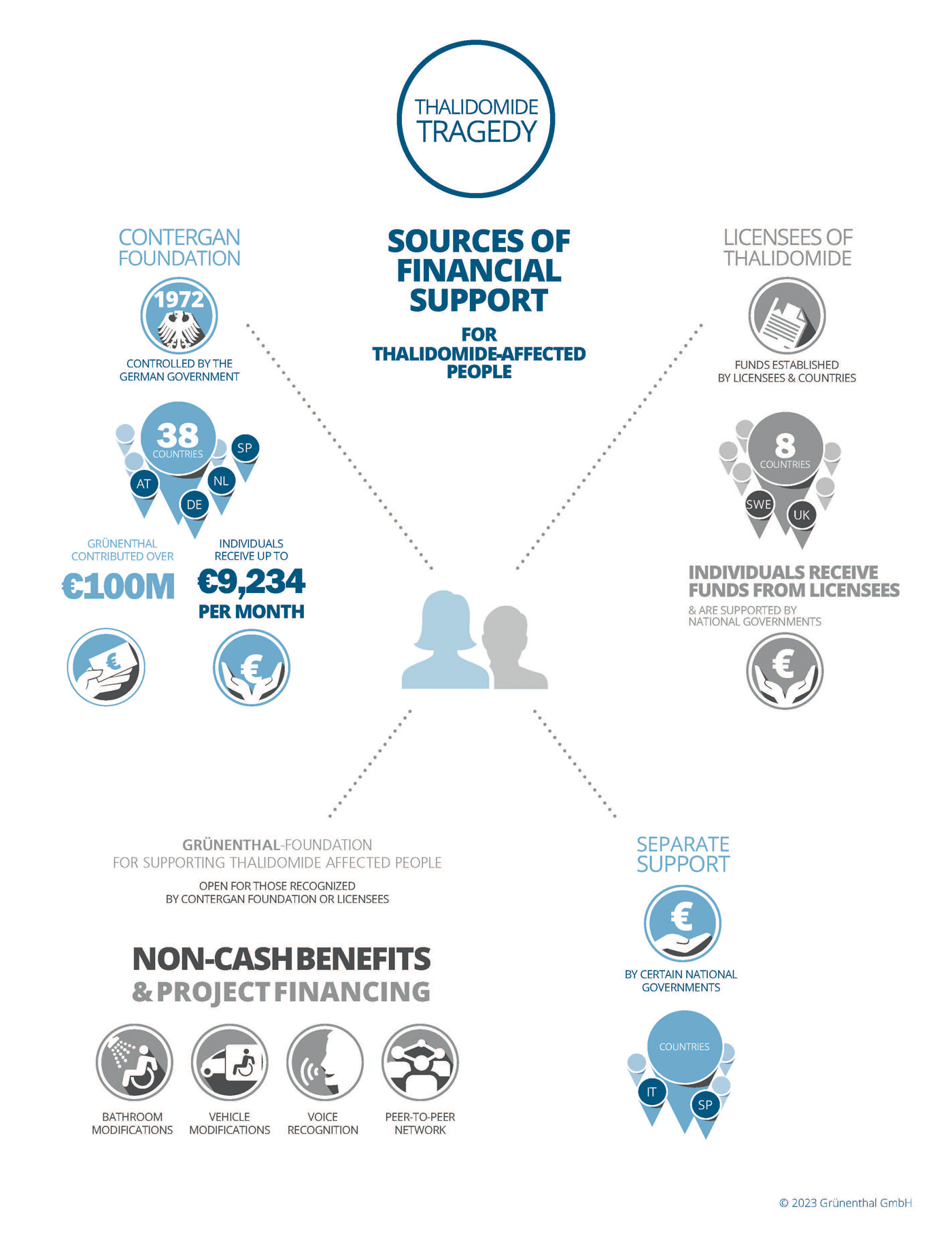
In 1972, the Contergan Foundation for Disabled People (Contergan Foundation) was established as a federal German foundation under public law. Since then, it has provided Thalidomide survivors in 38 countries with financial assistance including monthly pensions and individual payments.
The Foundation is overseen by the Federal Ministry for Family Affairs, Senior Citizens, Women and Youth and acts independently from Grünenthal.
To establish the Contergan Foundation, Grünenthal and the federal government each contributed 50 million euros (100 million German Marks at that time). In 2009, Grünenthal voluntarily paid an additional 50 million euros into the Contergan Foundation.
Visit the Contergan Foundation’s website for more information (German only)An international support system for Thalidomide survivors
Financial support programmes have been established in all of the countries where Thalidomide was marketed by Grünenthal or by its licensing partners at that time. This support is frequently backed by state programmes. Today, there are various forms of support available for people who were affected by products containing Thalidomide:
Historical background of the Thalidomide tragedy
The history of the Thalidomide tragedy is multi faceted and complex. It spans a time frame of over 70 years and affected individuals and their families in almost as many countries.
Thalidomide was sold as a sedative and sleep aid between 1957 and 1961. It was marketed by licensing and distribution partners of Grünenthal under different brand names including Contergan, Softenon and Distaval.
The Contergan Foundation estimates that around 10,000 children worldwide were born with deformities that can be attributed to Thalidomide. About half of them died at or shortly after birth.
Visit our Thalidomide website for more historical informationData and Facts
Data and Facts
Data and Facts
Data and Facts
The “Thalidomide Trials” against nine senior Grünenthal employees tried to answer the question of who was responsible for the tragedy. It was one of the most complex criminal proceedings in German legal history. After two and a half years, the trial was concluded in December 1970 without a verdict.
Parallel to the trial, the families of the affected people agreed on a settlement with Grünenthal, laying the cornerstone of today’s system of financial support.
The fate of the Thalidomide babies and the related court proceedings in Germany are still widely known and referred to as the “Thalidomide Scandal”.
Find out more about the historical background on our dedicated websiteIs Thalidomide still used today?
Thalidomide is only used today in specific circumstances to aid severe diseases such as bone marrow cancer and some autoimmune diseases. The manufacturing and use of the substance is subject to strict regulations.
Grünenthal no longer produces any Thalidomide-containing products. We have no connection to third parties who offer Thalidomide-containing products.
Find out more about the active substance of Thalidomide on our dedicated website