At our Mitlödi site, seven interconnected heat pumps now generate all production heat with green electricity, cutting energy use by 38%.
Grünenthal is a global leader in pain management.
Grünenthal is a global leader in pain management and related diseases. As a science-based, privately-owned pharmaceutical company, we have a long track record of bringing innovative treatments and state-of-the-art technologies to patients worldwide. Our purpose is to change lives for the better, and innovation is our passion. We are focusing all our activities and efforts on working towards our vision of a world free of pain.

Grünenthal at a glance – experience our virtual company showcase
Read about our key objectives and activities, as well as our recent business development highlights and financial performance. Get insights into our leadership team, strategy and financials, R&D & Pipeline, Product portfolio, Reliable Supply, Environmental & Societal Governance, and our Company culture and purpose.
Grünenthal Stories
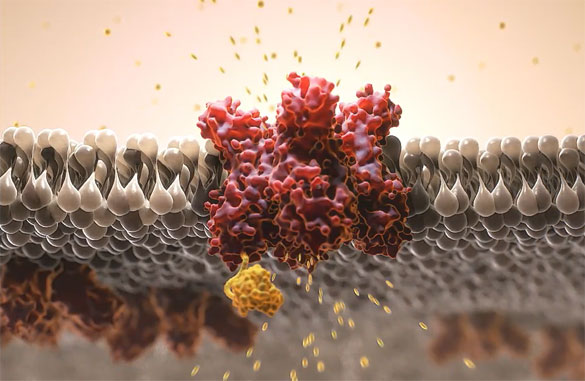
First-In-Human Trial with NaV 1.8 inhibitor

Nebido® licensing agreement
We have entered into a licensing agreement with Apotex Inc. to bring Nebido® to patients in Canada.
Searchlight Pharma, Apotex’s branded medicine division, will pursue marketing authorisation and, upon regulatory approval, market and distribute the brand in Canada, one of the largest pharmaceutical markets in the world.

Grünenthal Report 2024/2025
Grünenthal has a clear, bold vision: a World Free of Pain. In 2024, we took big steps forward in developing innovative medicines for patients living with pain worldwide. The latest Grünenthal Report provides information about our key objectives and activities, as well as our recent business development highlights and financial performance.

Responsibility Report 2024

Careers
Working at Grünenthal means experiencing the impact you can have on the results we achieve and the lives of the patients we serve.
Join forces.
Make an impact.
Innovate for a world free of pain.
Visit our careers website


















