
Explore our recent stories to see what drives us
Partnership to develop innovative cell therapy for chronic low back pain
Next generation pain therapies
“We are leveraging promising new therapeutic modalities and addressing high unmet medical needs.”
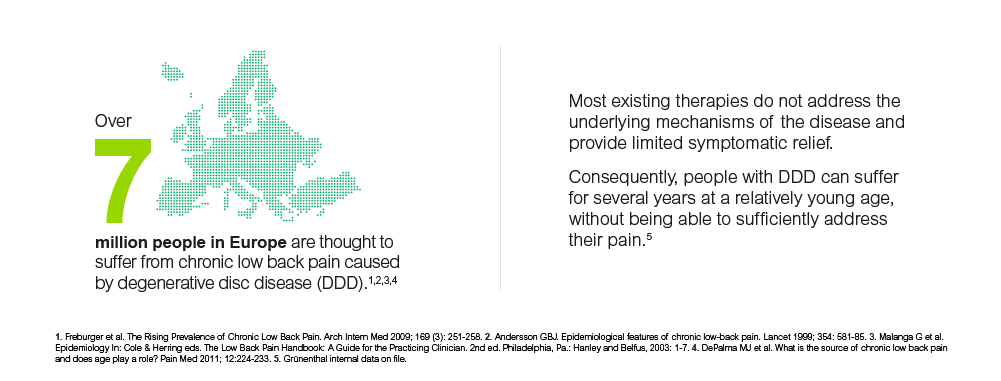
Over 7 million patients in Europe are thought to suffer from chronic low back pain caused by degenerative disc disease1,2,3,4, a condition that can cause significant pain and finally result in loss of function5. MPC-06-ID, a cell therapy candidate, is being developed for patients who have exhausted conservative treatment options. In a previous U.S. Phase II trial, Mesoblast demonstrated that a single intra-discal injection of MPC-06-ID using a unit dose of 6 million allogeneic mesenchymal precursor cells (MPCs) leads to meaningful and durable improvements for patients in pain intensity and functionality for at least three years6. A first Phase III trial is ongoing in the US, a second trial for Europe will follow.
Grünenthal’s CEO Gabriel Baertschi said: “This is an exciting day for Grünenthal. Cell-based therapies can potentially deliver meaningful lasting improvements to patients beyond symptomatic treatment by maintaining or even restoring physiological function. By teaming up with Mesoblast, we are diligently executing our strategy: leveraging promising new therapeutic modalities and addressing patients with high unmet medical needs. This is an important next step in working towards our vision of a world free of pain.”


Mesoblast CEO Dr Silviu Itescu stated: “We are very pleased to enter into this strategic partnership with Grünenthal. Together we plan to bring an important new class of therapy for pain management to the many patients suffering with degenerative disc disease. This partnership is in line with our corporate strategy to team up with best in category commercial leaders to maximise market access for our innovative cellular medicines for the treatment of patients suffering from debilitating or life-threatening inflammatory conditions.”
Under the partnership, Grünenthal will have exclusive commercialisation rights to MPC-06-ID for Europe and Latin America. The partners have also agreed on an overall development plan for MPC-06-ID to meet European regulatory requirements. As part of this plan, the companies will collaborate on the study design for a confirmatory Phase III trial in Europe.
“Together we plan to bring an important new class of therapy for pain management to patients.”
About chronic low back pain due to degenerative disc disease (CLBP)
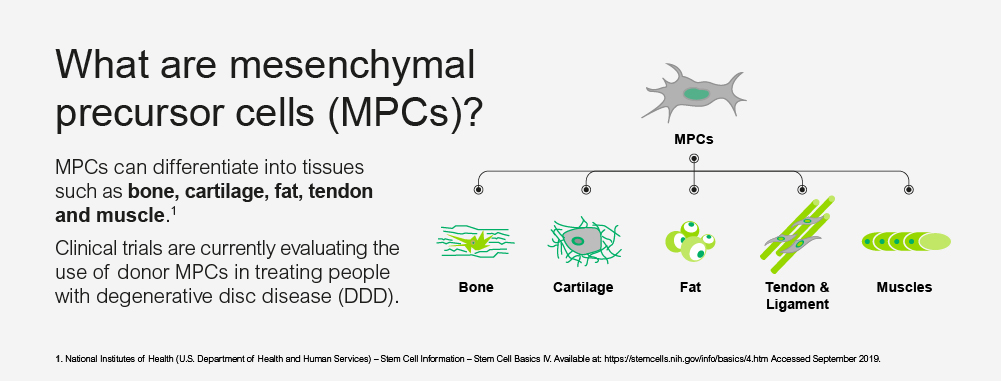
Over 7 million patients in Europe are thought to suffer from CLBP caused by degenerative disc disease 7,8,9,10, a disease which involves inflammation and degeneration of the intervertebral discs due to various factors like age, trauma or genetic pre-disposition. The lack of ‘cushioning’ as one of the major physiological functions of the disc in turn can result in spinal instability, mechanical stress and bony changes of the spine which finally cause significant pain and loss of function11. In addition, the inflammation of the disc can cause severe pain, which is poorly responsive to systemic pain treatment12. Most existing therapies do not address the underlying mechanisms of these changes and provide limited symptomatic relief. Patients would typically suffer for several years already at a relatively young age, without being able to sufficiently address their pain13. Invasive therapies, including surgeries like spinal fusion, are sometimes a last resort for these patients, however, the limited evidence of their long‐term effects remains a matter of concern14. If clinical trial results from Phase II are confirmed, MPC-06-ID could offer a new treatment option to patients otherwise considered unresponsive to conservative therapy, which can provide relief for at least 3 years and aims to retain the natural function and anatomy of the disc. MPCs offer the possibility to support and promote the existing regenerative potential inherent in host tissues and are expected to deliver superior long term outcomes compared to purely symptomatic treatments15.
1 Andersson GBJ. Epidemiological features of chronic low-back pain. Lancet 1999; 354: 581-85
2 Freburger et al. The Rising Prevalence of Chronic Low Back Pain. Arch Intern Med 2009; 169 (3): 251-258
3Malanga G et al. Epidemiology In: Cole & Herring eds. The Low Back Pain Handbook: A Guide for the Practicing Clinician. 2nd ed. Philadelphia, Pa.: Hanley and Belfus, 2003: 1-7
4 DePalma MJ et al. What is the source of chronic low back pain and does age play a role? Pain Med 2011; 12:224-233
5Rider SM et al. Spine Surg Relat Res. 2018 Apr;3(1):1-11
6 https://www.mesoblast.com/product-candidates/spine-orthopedic-disorders/chronic-discogenic-low-back-pain; https://www.mesoblast.com/clinical-trial-results/mpc-06-id-phase-2
7 Andersson GBJ. Epidemiological features of chronic low-back pain. Lancet 1999; 354: 581-85.
8Freburger et al. The Rising Prevalence of Chronic Low Back Pain. Arch Intern Med 2009; 169 (3): 251-258
9 Malanga G et al. Epidemiology In: Cole & Herring eds. The Low Back Pain Handbook: A Guide for the Practicing Clinician. 2nd ed. Philadelphia, Pa.: Hanley and Belfus, 2003: 1-7
10 DePalma MJ et al. What is the source of chronic low back pain and does age play a role? Pain Med 2011; 12:224-233
11Rider SM et al. Spine Surg Relat Res. 2018 Apr;3(1):1-11
12 Nguyen QT et al. ACS Biomater Sci Eng. 2017 Nov;
13 Grünenthal internal data on file
14 Gibson AJN, Waddell G. Spine 2005; 30: 2312– 2320
15 Fernandez-Moure J, et al. SAGE Open Med. 2018 Mar;6:2050312118761674